My research focuses on the development of statistical methodology for censored survival data, group sequential methods for the design and analysis of clinical trials, and the analysis of longitudinal data. I have served as a special government employee (SGE) and advisor to the US FDA for the past 15 years. In this capacity, I have serve on various advisory committees including BRUDAC, Cardio Renal, Drug Safety, and EMDAC, among others. I am also active in working with industry sponsor to design and monitor international multisite clinical trials. I have served as member or chair on the independent data and safety monitoring committee (iDMC) for over 50 clinical trials. As a general rule, my methodologic research is motivated by applications stemming from a multitude of clinical disciplines including Alzheimer’s disease, cancer, renal disease, and environmental science. Below are brief descriptions of some of the current collaborations that motivate my methodologic work. My Google Scholar page is available here.
Statistical Methods for Early and Late Stage Clinical Trials
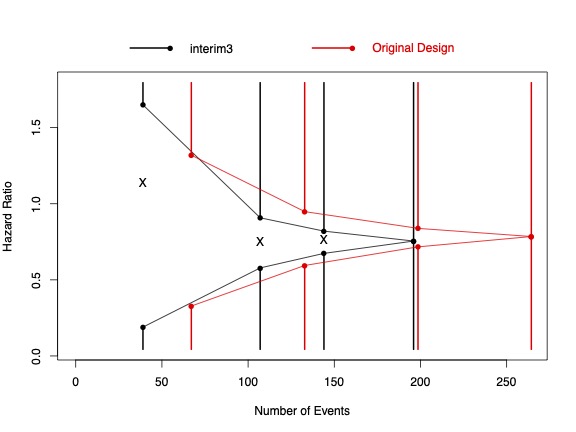
This research focuses on the development of statistical methods for efficiently conducting early and late phase clinical trials through the use of interim testing and adaptive procedures. Specific collaborative projects include trial design and recruitment science for MCI and AD trials (Josh Grill, Psychiatry, UCI), estimation of maximally tolerable doses for new experimental interventions (Leslie Randall, Medicine, UCI), censoring robust survival estimators under model mis-specification (John Kittelson, Biostatistics, U. of Colorado), and sequential testing of weighted survival and longitudinal data (Scott Emerson, Biostatistics, U. of Washington). For further information on this research and to obtain software for designing, analyzing, monitoring and reporting sequential clinical trials, visit www.RCTdesign.org.
Robust and Flexible Statistical Models for Environmental Epidemiology Studies
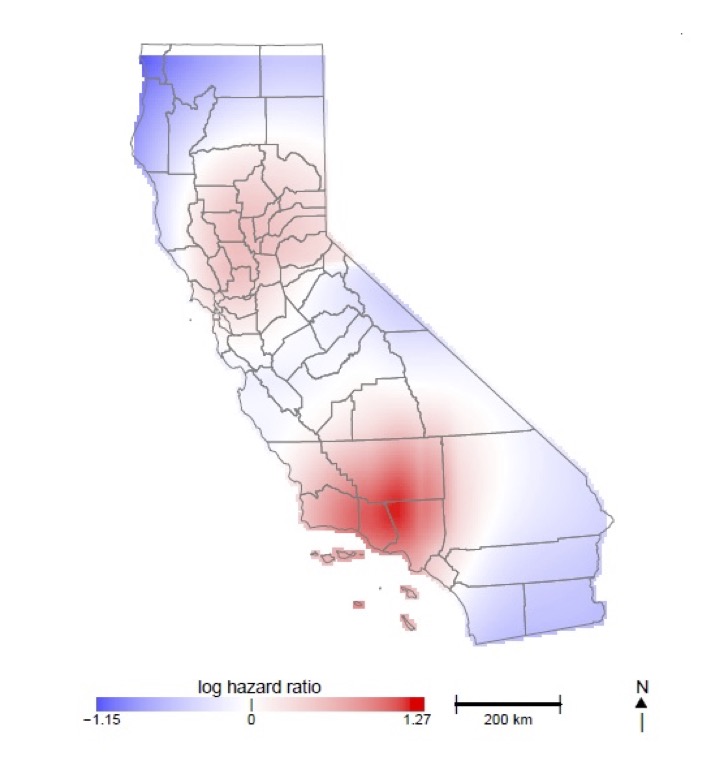
In this research we are developing novel statistical methods to flexibly estimate the association between spatially and/or temporally correlated environmental exposures and the risk of clinical outcomes in humans. A broad theme of this research is that developed methods focus on valid estimation and inference over a wide-range of statistical assumptions. Specific collaborative projects include estimation of acute air pollutant effects using case-crossover designs with heavily imbalanced data (collaboration with Ralph Delfino, Epidemiology, UCI), non-parametric tests of hormetic environmental toxin dose-response curves (collaboration with Scott Bartell, Public Health, UCI), and incorporating spatially smoothed environmental exposure into models for censored survival data (collaboration with Veronica Vieira, Public Health, UCI).
Flexible Joint Longitudinal Survival Models
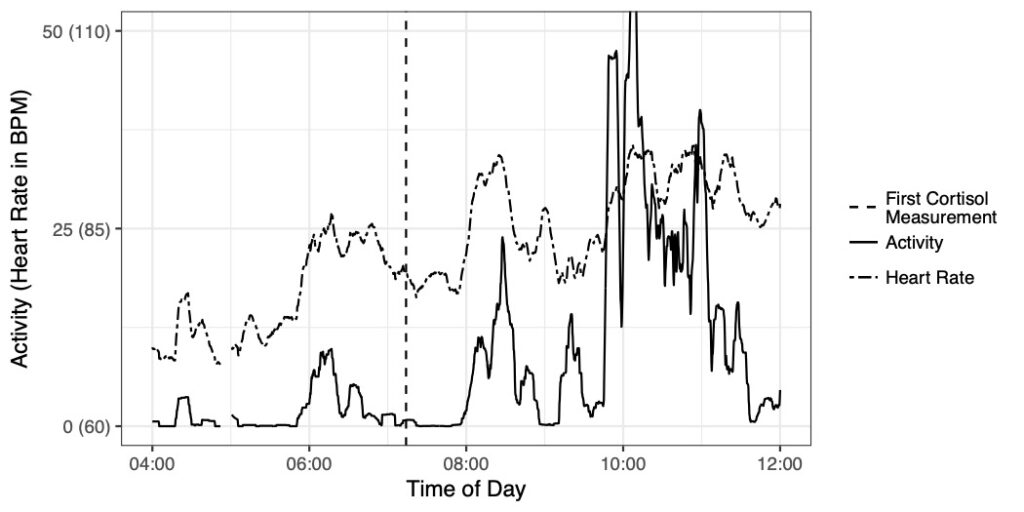
In this research we are developing Bayesian longitudinal-survival models to estimate the association between longitudinally measured biomarkers and the time to clinical outcomes. Specific collaborative projects include the development of joint longitudinal survival models for estimating stress events as a function of biophysical parameters such as heart rate and heart rate variability (collaboration with Pathik Wadhwa, Sonja Entringer, Cladia Buss, and Ben Ward) and the association between within subject volatility in nutrition index markers and the risk of death among end-stage renal disease patients (collaboration with Babak Shahbaba and Tracy Holsclaw, Statistics, UCI and Kam Kalantar, Nephrology, UCI).